

Iso 14971 iso#
The main body of the ISO 14971 standard is surprisingly scant with only 18 pages plus 3 annexes. Evolution of ISO 14971 and the elevation of ISO/TR 24971:2020 Read our blog post to get up-to-speed on changes in ISO 14971:2019. This version replaces ISO 14971:2007 and EN ISO 14971:2012 and while no tectonic shifts have occurred in the risk management process, there are important changes and updates to be aware of. The most recent version – ISO 14971:2019 – was published by ISO and as EN ISO 14971:2019 by CEN/CENELEC. In all cases, the goal is to analyze, evaluate, control, and monitor the risks associated with each life-cycle stage. The intent of ISO 14971 is to define a standard process for identifying risks associated with medical devices at all stages in a device’s life cycle, from product design to procurement to production and postmarket use.

Both are copyrighted documents and you can purchase them online from ISO.org and other sources.
If you are just getting started implementing risk management for your company, purchase the ISO 14971:2019 standard and its guidance ISO/TR 24971:2020, which provides support to implementing risk management. Instead, they defer to ISO 14971, the global standard for medical device risk management.
Iso 14971 how to#
The role of ISO 14971įortunately, national governments have not created their own unique guidelines telling you how to how to perform risk management. Likewise, Japan, Canada, Australia, Brazil, and all other major markets require the application of risk management, which is either referenced in their national regulations or ISO 13485:2016. Europe requires it in the Medical Device Regulation (MDR 2017/745). The US FDA mandates it in the Quality System Regulation (21 CFR Part 820). For that reason, risk management is not optional – it is a regulatory requirement worldwide. Simply put, we have a collective interest in ensuring that medical devices are safe and effective.

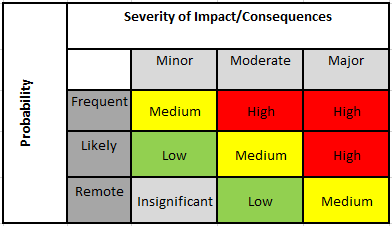
Risk management – the systematic application of management policies, procedures, and practices to the tasks of analyzing, controlling, and monitoring risk. Risk– the combination of probability of occurrence of harm and the severity of that harm. Before we get into that, let’s step back and talk about the regulations and standards that dictate how you should approach risk management.įor medical devices, risk and risk management are defined as: Fortunately, there is a systematic process you can establish to analyze, evaluate, control, and monitor risks. The thing that makes risk management tricky is that we often don’t have enough real-world data to accurately quantify risks, especially for new devices. Part 4: Risk management review, reporting and post market planning.Part 3: Risk control and risk management tools.Part 2: Risk management planning and conducting a risk analysis.
Iso 14971 series#
This is a four-part series on risk management.


 0 kommentar(er)
0 kommentar(er)
